Join the CEEC mailing list, or join the EChem seminars listserv to only receive alerts about electrochemical energy related campus seminars.
CEEC is affiliated with the Columbia University Earth Institute and resides in the Engineering School.
Electrochemical Energy
Renewable energy sources offer a sustainable solution to meet the energy needs of the future. To overcome the intermittency of solar and wind we are focusing on strategies to address energy storage and conversion using batteries, fuel cells, and electrolyzers in transformative ways.
Addressing the Global Energy Crisis
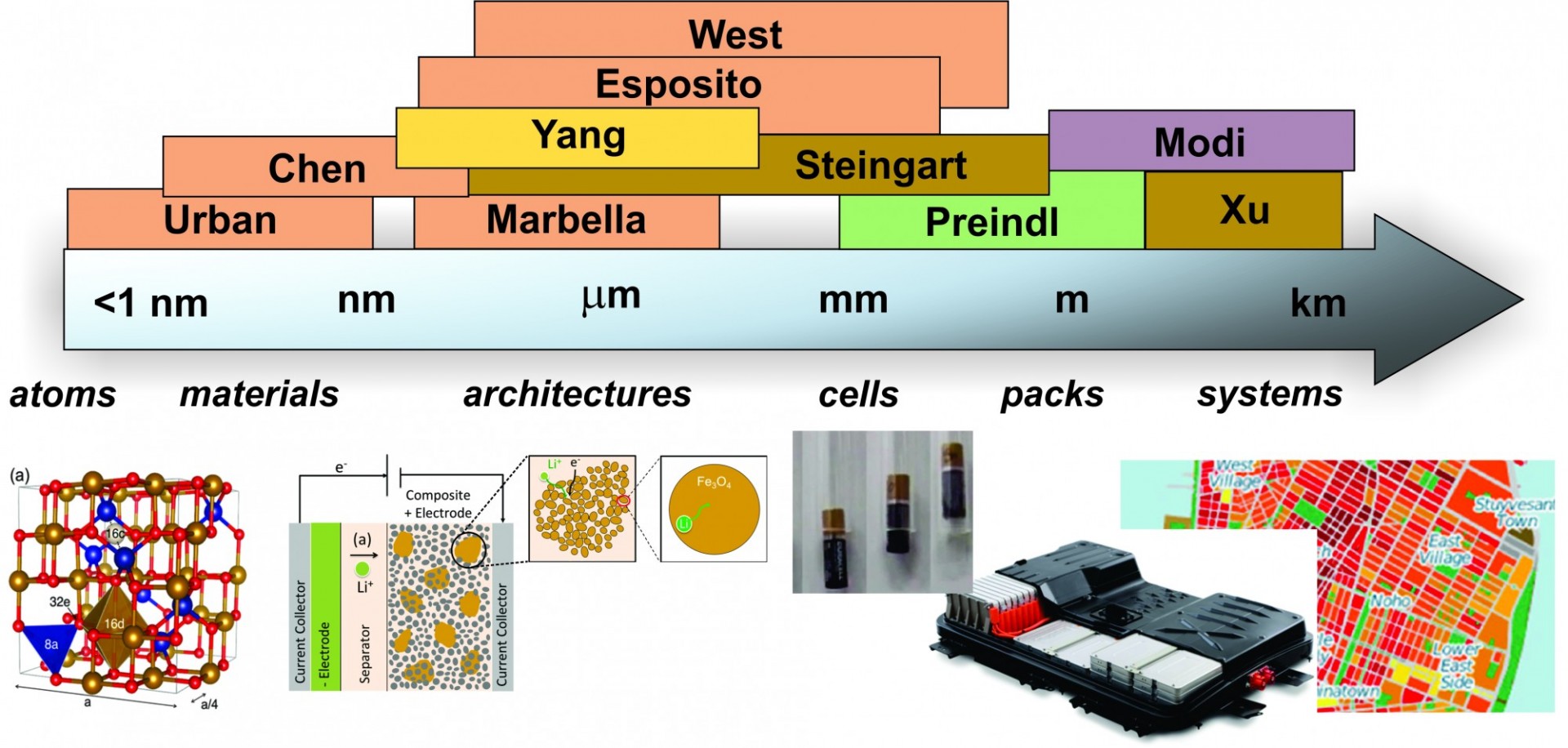
The Columbia Electrochemical Energy Center (CEEC) is using a multiscale approach to discover groundbreaking technology and accelerate commercialization. CEEC joins together faculty and researchers from across the School of Engineering and Applied Sciences who study electrochemical energy with interests ranging from electrons to devices to systems. Our industry partnerships enable the realization of breakthroughs in electrochemical energy storage and conversion.
News
Energy partnerships and deep expertise make the Columbia Electrochemical Energy Center a hotbed of activity for entrepreneurs advancing the clean e
Go behind the scenes at one of the Columbia Electrochemical Energy Center’s labs, where researchers are developing the next generation of batteries

We are proud to present the Junior Faculty in Batteries: The Next Generation of Energy Storage virtual symposium that is taking place on December 1
